New research information taken from the German Patient Organization

New research information taken from the German Patient Organization, published on 8.11.2025.
Here is the link.
https://tay-sachs-sandhoff.de/2025/11/08/neue-studien-forschungen-und-projekte-lassen-hoffen/
New studies, researches and projects give hope
We were very pleased to be able to present some news and interesting facts in the medical part of our jubilee congress on the occasion of our tenth anniversary. We would like to thank all our speakers for this. This is followed by an update on the most important innovations related to the state of medical research. We used Saturday to inform our families, doctors and therapists about the latest research on gangliosidosis.
Our medical advisory board, Dr. Eugen Mengel, founder and CEO of SphinCS, opened the medical part of our annual conference. And he had exciting news with him. Three therapeutic approaches are newly emerging at once – substrate reduction, Chaparone effect and enzyme replacement therapy. In addition, he is fascinated by an exciting research project by scientists from Freiburg.
But first things first: The pharmaceutical company "Azafaros" will launch a clinical trial worldwide in the coming weeks to approve the active ingredient it has developed nizubaglustat for GM1, GM2 and Niemann-Pick type C as a drug. This is an 18-month, double-blind, randomized, placebo-controlled, multicenter, phase 3 study that evaluates the safety and efficacy of nizubaglustat (AZ-3102) in late infantile and juvenile forms of GM1 gangliosidosis or GM2 gangliosidosis, as well as late infantile and juvenile forms of Niemann-Pick disease type C. Nizubaglustat is a substance that is taken orally once daily. The potential for clinical effects in GM1/GM2 gangliosidosis is supported by a non-clinical model of Sandhoff's disease, in which nizubaglustat prolonged survival and improved performance at behavioral endpoints.
Who is eligible for this study?
In late infantile and juvenile patients with GM1 and GM2 between the ages of 4 and 20 years, the first neurological symptoms must appear between the first and tenth year of life. A SARA test must be possible and there must be no very serious swallowing disorders. SphinCS in Hochheim is the only institute in Germany that has been submitted as a test center for the study. In Switzerland, the study test center is the Department of Neurology and Neuropediatrics of the University Hospital Bern. Our medical advisory board, Dr. Eugen Mengel, had many reports on the state of research on GM1 and GM2 gangliosidosis.
Aloxistatin has a completely different effect. It belongs to the guards in the broadest sense. The so-called chaperones attach to improperly folded enzymes and stabilize the structure of the enzyme. Thanks to this, it is possible to decompose the substrate in the lysosome again. However, according to Mengele, aloxistatin is not a true chaperone effect. This is an "enzyme enhancement", but for a different reason than chaperone. Still, aloxistatin can increase, improve, or even restore ß-Gal or Hex A activity.
Aloxistatin is a well-known substance - with few side effects and safe. D-Orphan, a small pharmaceutical company from Switzerland, rediscovered it by accident while looking for a cure for MPS. Originally, it was considered only for GM1, because a common mutation in GM1 is typical for improper enzyme folding. However, there are also mutations in GM2 that, according to Mengele, would cause the enzyme to be incorrectly formulated. For anodization to be effective, the patient must have at least one such mutation.
In order to prove this, Dr. Mengel drew up a study protocol, which had to be approved by the Federal Institute for Drug Safety (BfArm) and the ethics committee. A hearing at the BfArm is scheduled for this year. If all goes well, of course, it is still necessary to procure the fabric.
Good news comes from the Japanese pharmaceutical company JCR, which is working on a method to get enzyme replacement therapy across the blood-brain barrier. In enzyme replacement therapy, the enzyme necessary for the breakdown of the lysosome is introduced from the outside. In our clinical pictures (GM1 and GM2), this has so far failed at the blood-brain barrier. Research can now continue after a pharmaceutical company has found an investor and can thus produce the enzyme for basic research on a large scale.
In addition, Dr. Eugen Mengel reported on the surprising research approach of a team of scientists from Freiburg, who took a detailed look at the course of the GM2 disease and defined a kind of vicious circle of inflammation and neuronal damage and its cause. The researchers also showed that this vicious circle can be broken by replacing the microglia that the enzyme lysosome supplies to neurons with other, peripherally derived cells similar to microglia. With these "new" ones, balance in the brain can be restored, and neurodegenerative damage can be reversed. It's extremely exciting to see how this research continues, says Mengel.
Surface N-acetyl-L-leucinu a ataxie
PD Dr. Tatiana Brémová-Ertl was a guest speaker at our family conference for the first time in 2017. We have received another research update from Priv.-Doz. Tatiana Brémová-Ertl, MD, PhD, from the Department of Neurology and Neuropaediatrics, University Hospital Bern, Switzerland. Brémová-Ertl is the deputy director of the local Center for Rare Diseases and the head of the counseling center for neurometabolic fields.
First of all, your lecture was devoted to the topic of ataxia, which causes limitations especially in our adolescent and adult patients. The term ataxia comes from Greek and means disorder, irregularity. This term includes disorders of movement coordination, sequences, fine control of individual movements, and interactions of complex movement sequences. Brémová-Ertl explained the individual forms of ataxia, how they arise and what symptoms they lead to. She also explained the evolution of scores, such as the SARA score, which can be used to measure the effectiveness of ataxia-based drugs. SARA stands for "Ataxia Rating and Rating Scale".
She then provided us with an update on the situation of the active ingredient N-acetyl-L-leucine (NALL). Tatiana Brémová-Ertl was closely involved in the development of this active ingredient at the Vertigo Center of the University Hospital in Munich, Germany. From the very beginning, our self-help group has been involved in individual trials of treatment with a drug approved in France for dizziness and migraine.
Tatiana Brémová-Ertl spoke on this topic for the first time in 2017 at one of our conferences. Initially, the aim was to demonstrate efficacy for Niemann Pick Type C and, at our suggestion, also for Tay-Sachs and Sandhoff in individual treatment studies, in order to subsequently optimize and further develop the active ingredient. Thus, N-acetyl-DL-leucine became N-acetyl-L-leucine (NALL), which is used today.
N-acetylation creates a premolecule that, according to Bremen-Ertl, is very efficiently absorbed into cells. The high concentration of L-leucine in cells is therapeutic and improves the energy metabolism of cells. N-acetyl-L-leucine has a neuroprotective effect and prevents nerve loss. The drug has already been approved for Niemann-Pick type C in the US. The pharmaceutical company Intrabio has submitted an application for recognition of NALL in the GM2 indication. This was rejected, but with the option to record "patient pathways" to obtain extended GM2 approval.
Basic research and mouse model for gene therapy
Professor Dr. Wolfgang Baumgärtner spoke for the first time at one of our conferences. Prof. Baumgärtner conducts research at the Veterinary University of Hannover. There he was for many years the head of the Department of Pathology and the head of the Department of Diagnostics and the head of the research unit of neuropathology and neuroimmunology. From 2006 to 2009, he investigated the characterization of the molecular defect of GM1 gangliodosis in Alaskan huskies as part of the DFG research fellowship. Another DFG project focused on correcting a genetic defect of GM1 gangliodosis in Alaskan huskies using nucleases from zinc fingers. Prof. Wolfgang Baumgärtner conducts basic research on GM1 in Hannover.
Baumgärtner gave us an exciting insight into his basic research: Naturally occurring genetic defects in the Glb1 gene exist not only in humans, but also in dogs, cats, cows, sheep, American black bears and other animals. Artificially induced (knock out) in various mouse models. The clinical, morphological and biochemical features of the diseased Alaskan Huskies were similar to those of the late infantile/juvenile form (type 2) of human GM1 gangliosidosis. Knock-out mice, on the other hand, mimic the adult form of GM1 gangliosidosis in humans in terms of histological and immunohistochemical findings.
His current project is "A novel gene therapy strategy for the correction of GM1 ganglioside in a mouse model with special attention to the early and late stages of the disease".
Baumgärtner also reported on his preclinical studies on the substrate inhibitor Sinbaglustat. Long-term treatment of Glb1 knock-out mice with sinbaglutat resulted in delayed onset and slowing of clinical disease progression due to reduced neuronal accumulation of GM1. However, neuronal accumulation of GM1 could only be reduced to a limited extent.
The therapeutic approach consists in inhibition of enzymes involved in gangliosidosis by the sugar imino (aza sugar) sin baglustat. It is a potent inhibitor of extralysosomal glucosylceramidase beta 2 (GBA2, glucocerebrosidase) and a weak inhibitor of glucosylceramide synthase (GCS). Unfortunately, sinbaglustat, which could also be used for GM2, is not currently being monitored by the Swiss pharmaceutical company Idorsia due to cost.
Our "8-in-1" and PRADO studies
Dr. Hannah Arnold was also a guest at our family conference for the first time. Hannah Arnold, MD, PhD, gave a first overview of the Prado-Stduie (Project Adult Onset) project, which has been running since March 2025. The aim of the PRADO study is to systematically record psychological symptoms or psychiatric comorbidities in patients with Tay-Sachs disease and Sandhoff disease. Specifically, it is also the role of the cerebellum. This is because cerebellar function disorders are associated with the development of acute psychoses. GM2 gangliosidosis could serve as a model disease for other diseases associated with cerebellar dysfunction.
Inclusion criteria:
• juvenile or adult form of GM2 gangliosidosis
• Age at least 12 years
A total of 17 subjects were included in the study:
• 3 subjects with M. Sandhoff
• 14 subjects with Tay-Sachs disease
. Age: 16 years to 61 years old
. 6 juvenile subjects and 11 adult subjects
Cognitive tests and a CCAS test, which tests cerebellar function, have been performed. In addition, short psychiatric interviews were conducted to ask about the most important psychiatric symptoms, both present and past.
First results:
• No relevant cognitive deficits, no signs of cerebellar damage were found in patients with GM2 gangliosidosis examined
• Psychiatric diagnoses were most often found in anxiety disorders, depression and psychosis
Dr. Eugen Mengel provided an update on the "eight-in-one study" that our self-help group started together with SphinCS five years ago. This is a registry and natural follow-up study for all GM1 and GM2 gangliosidosis. In the meantime, 84 patients have been admitted. Of the eight gangliosidosis, seven are represented by:
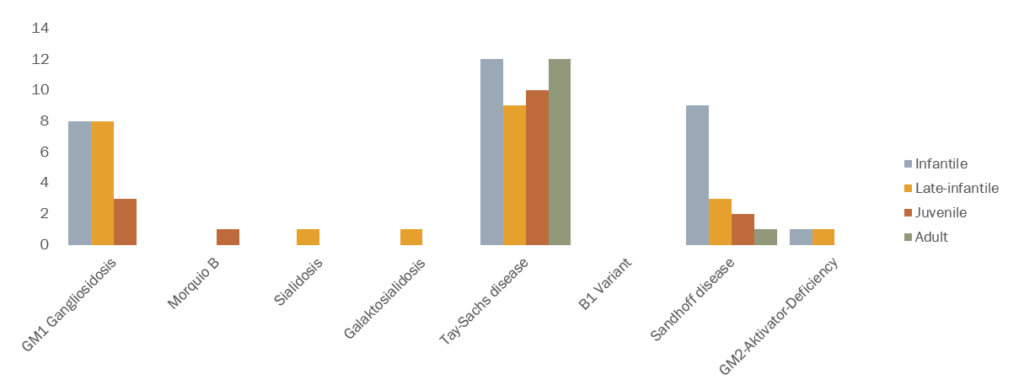
Eugen Mengele's conclusion after five years of the "8-in-1" registration study:
• These data would support the need for effective therapies.
• Early diagnosis is important, for this you need to listen to parents, but neonatal screening is also important.
• An incredible number of 84 patients
• Prado helps to better understand mental illness
• Late-onset GM2 patients: Suffer from disease of the nerves that supply the muscles and cerebellar disorder
. Drug developers know about us

